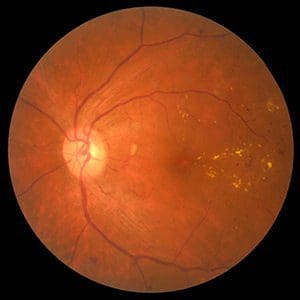
PEDF Mimetics
Medical Need
Diabetes is generally described as the largest epidemic of the 21st century; in 2015, 9.4% of the U.S. population had diabetes, totaling 30.3 million adults.
Diabetic retinopathy (DR) is a leading cause of blindness, with a prevalence of 35% of diabetics. Existing injectable products cannot be used early-stage, fail to elicit a response in ~30% of patients, and often fail to prevent long-term retinal thinning. The total cost of U.S. retinal vision loss was estimated at $8.7 billion in 2013.
Target Market
Globally, there are ~93 million people with DR; over eight million of these patients are in the United States.
The National Eye Institute projects that by 2030, 11.3 million Americans will have DR. Many of these patients require early treatment and prefer an eye drop over regular intraocular injections.
Target Patient Segments
- Patients with early-stage disease
- Patients who prefer drops over intraocular injections
- Patients who have failed anti-VEGF therapy
Competitive Advantages
- Current treatments fail to address early-stage retinal damage. Skyran will test Spx81-5 in early disease.
- Some patients respond transiently and become resistant to anti-VEGF therapies, while approximately 30% of patients do not respond at all. PEDF therapy may be useful in these patients.
- Anti-VEGF treatment fails to prevent disease progression; retinal thinning is evident over 2-5 years
- Anti-VEGF therapies require regular ocular injections (every 4-6 weeks) in a doctor’s office. Our data-to-date show the utility of Spx81-5 in a drop formulation.
STRONG INTELLECTUAL PROPERTY
-
Issued U.S. patent
-
Filed 9/12/2014, issued 4/4/2017
-
Composition of matter for lead compound and five analogs
-
Includes administration by eye drop, foam, gel, or injection
-
Covers use in diabetic retinopathy, retinopathy of prematurity, glaucoma, and more
-
Divisional patent filed 4/3/2017
-
Includes 16 additional analogs

Issues With Current Therapies:
Requires Injection
Into Eye
Inability to Treat Early-Stage Disease
Declining Effect
Over Time
Failure
Rate
%
Our Technology
Skyran Biologics, Inc.
Skyran Biologics is developing a small protein therapeutic, delivered as an eye drop, a mimetic of the active binding region of Pigment Epithelium-Derived Factor (PEDF), to treat diabetic retinopathy (DR), a leading cause of blindness.
Skyran’s preclinical data show that an eye drop formulation of this molecule delivers the active ingredient to the retina and can significantly reduce or halt retinal degeneration, thereby reducing or eliminating loss of sight caused by diabetic retinopathy.
PEDF is a 418-amino acid polypeptide in the serpin gene family secreted by the retinal cells.
The protein is widely recognized for its neuroprotective, anti-inflammatory, and anti-angiogenesis activities in the retina and other tissues and may be deficient in diabetic patients. Skyran has isolated a 17-mer minimum active region of PEDF and used a series of conservative peptide modification strategies to obtain several novel, more active PEDF mimetics.
Spx81-5, the lead PEDF mimetic, has increased biological activity over the native fragment.
Spx81-5 shows no evidence of toxicity in preliminary studies, and adopts a well-defined alpha helical structure making it a pharmaceutically attractive molecule. Skyran has formulated Spx81-5 for topical use as an eye drop that shows bioavailability in the Ins2Akita genetic mouse model of DR and in the non-human primate eye. Spx81-5 and several analogs are protected by U.S. patent 9,611,314, filed September 12, 2014 and issued April 4, 2017.
Spx81-5 Is Active When Delivered By Eye Drop
Immunolabeling studies show that Spx81-5 achieves widespread deposition in the mouse retinal. Additional studies demonstrate that Spx81-5 administered as an eye drop reaches significant levels in the monkey vitreous.